Clostridioides difficile is a Gram-positive, strictly anaerobic, spore-forming bacterium responsible for nosocomial and community-acquired intestinal infections. One of the major problems associated with these infections is the frequent occurrence of recurrences: in 15 to 25% of cases after a first episode, often due to a relapse linked to the same bacterial strain. These relapses are linked to C. difficile‘s ability to persist in the digestive tract, notably in the form of spores, and possibly biofilm. Although there is as yet no formal proof of the presence of C. difficile biofilms in vivo, several clues support this hypothesis. In the laboratory (in vitro), C. difficile can form biofilms, the biomass of which varies from strain to strain.
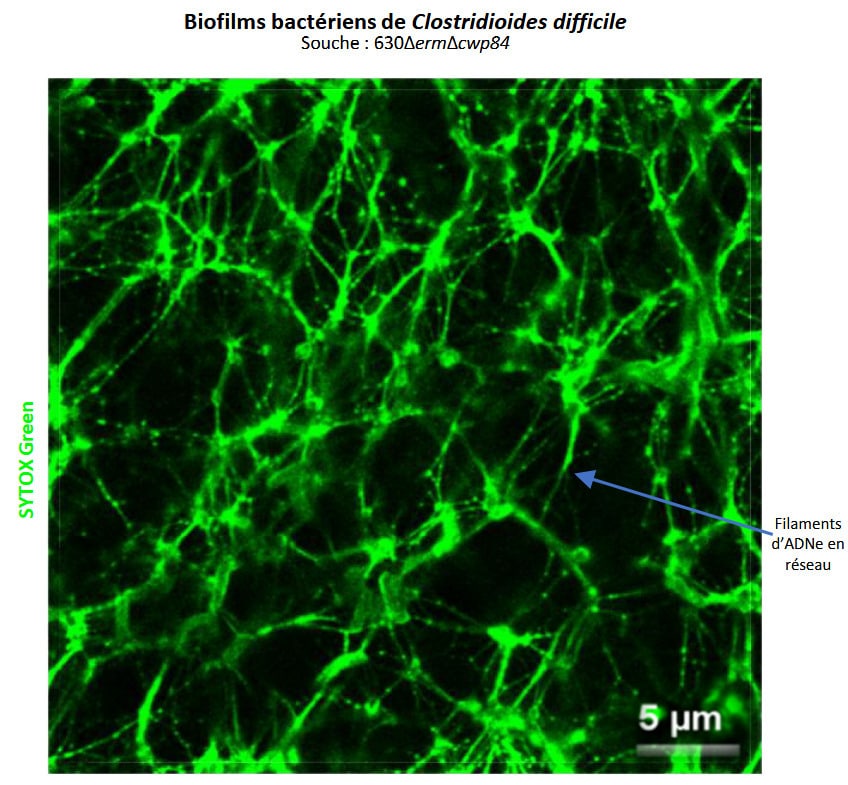
In this study, we characterized the architecture and matrix of biofilms produced by four strains of C. difficile: clinical strain R20291, laboratory strain 630∆erm, and two mutants (630∆erm∆fliC, non mobile; 630∆erm∆cwp84, with an altered surface layer). We measured the proportions of the main matrix components (polysaccharides, proteins, and extracellular DNA ([eDNA]) and characterized their architecture within the 48 h old biofilms:. The results obtained show that polysaccharides are present in greater quantities than proteins and above all eDNA. Analysis by confocal laser scanning microscopy cnfirmed the biofilms of the four strains tested are composed of dead bacteria at the base of the biofilm and live bacteria on the surface, that are embedded in a self-produced exopolymeric matrix. For the first time in C. difficile, fine eDNA filaments were observed within this matrix, forming a spider’s web structure involved in bacterial aggregation and biofilm cohesion. Despite the low proportion of eDNA in matrix, treatment with DNase leads to the total disappearance of these filaments and a wide dispersion of the biofilms of the 4 strains. Surface components such as polysaccharide II and lipoprotein CD1687 seem to colocalize with these filaments, depending on the strain, and could play a role in network stability. Other components, such as lipids and proteins, are also present in the matrix, but do not colocalize with eDNA.
We also explored potential mechanisms of matrix component release. In our conditions, autolysis does not appear to be the main driver of matrix release. . Structures reminiscent of extracellular vesicles were observed, but their nature remains to be confirmed. These results shed valuable light on the mechanisms involved in the formation and stabilization of C. difficile biofilms, which are likely to play an important role in intestinal persistence and relapses of infection.
Contact:
Links: