Natural genetic transformation is integrated into the cell cycle of pneumococcus.
The pathogenic bacterium S. pneumoniae, commonly known as pneumococcus, is capable of integrating DNA sequences present in the external environment into its genome. This natural transformation process, which is highly conserved in bacteria, contributes to their diversification by enabling intra- and inter-species gene exchange. It requires the induction of a specific physiological state known as “competence.” In pneumococcus, competence develops transiently in all cells of the population in response to stress during their multiplication phase. It is accompanied by a blockage of the division process, lengthening the cell cycle by a third and allowing the cells to complete the final stages of transformation without compromising the integrity of their genome. ComM, a membrane protein synthesized during competence, appears to be necessary and sufficient to induce this blockage. Thus, the transient division arrest dependent on ComM contributes to the high adaptive potential of pneumococcus by optimizing the transformation process without compromising cell viability.
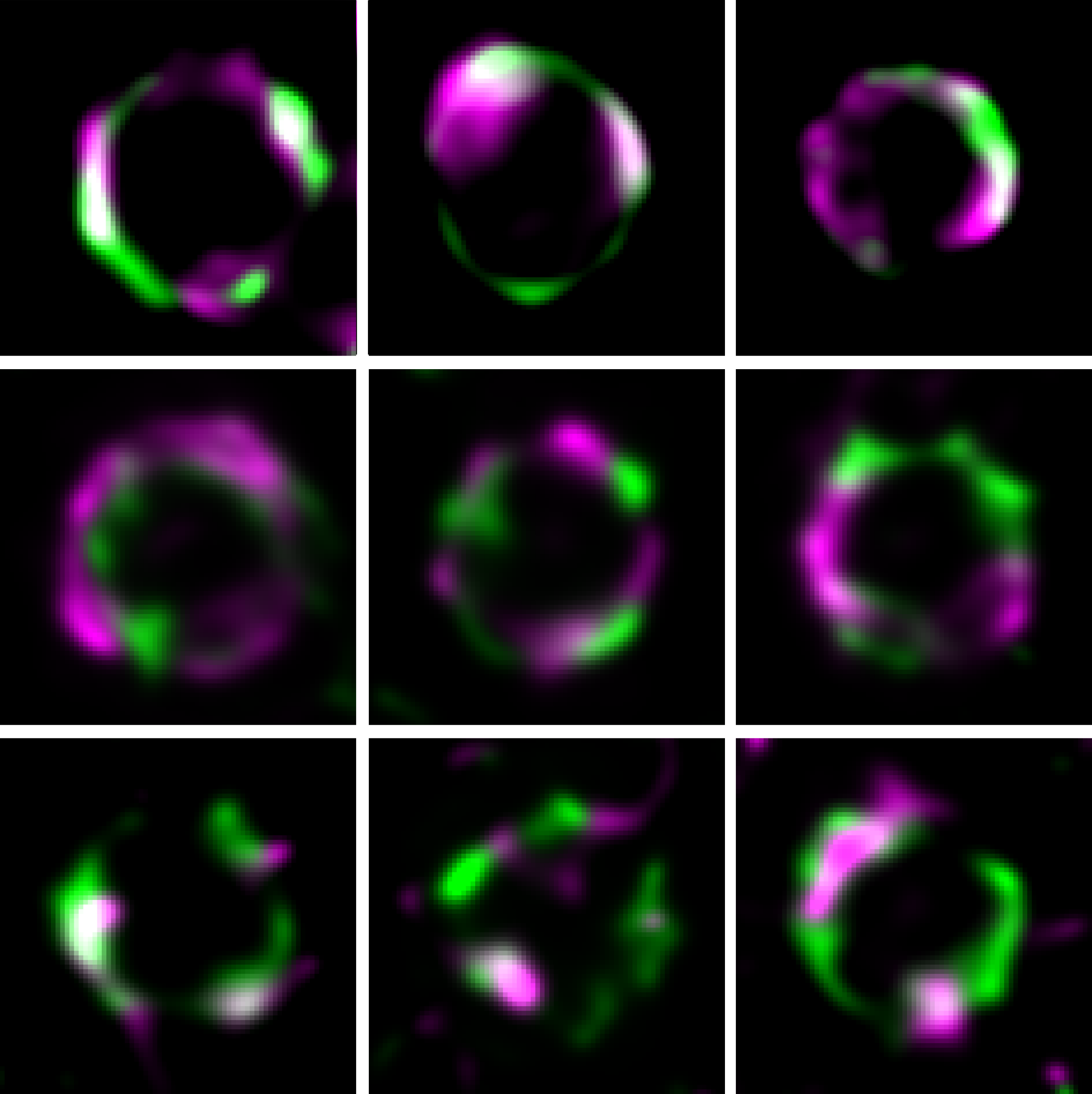
The study of protein dynamics during division using super-resolution microscopy reveals the mechanism behind pneumococcal division arrest during transformation.
In an article published in the journal Nature Communications, scientists have discovered the molecular mechanism of ComM-dependent division arrest. Using total internal reflection fluorescence (TIRF) microscopy, they show that ComM is a dynamic protein that reduces the speed of the septal wall synthesis complex, FtsW:PBP2x, and co-localizes with it. Their analysis also indicates that ComM interacts directly with DivIB, an activator of this complex. Remarkably, overproduction of this activator restores the speed of the FtsW:PBP2x complex and reverses the delay in division of competent cells. Thus, it appears that ComM reduces septal wall synthesis, and thereby division, by interfering with the activity of DivIB, a regulator of the FtsW:PBP2x complex.
This research represents a step forward in our understanding of how bacteria take control of the cell cycle in response to stress or to ensure the smooth running of differentiation processes.
Contact:
Links: