The gastrointestinal tract is a reservoir of opportunistic pathogens, where pathobionts take advantage of dysbiosis to proliferate in immunocompromised patients. Vancomycin-resistant enterococci (VRE) originate from the gastrointestinal tract, where their proliferation precedes dissemination into the bloodstream and can lead to systemic infection. Understanding the mechanisms responsible for resistance to intestinal colonisation by VRE is essential for infection control. Few studies have identified the commensal bacteria that enhance resistance to VRE colonisation of the microbiota.
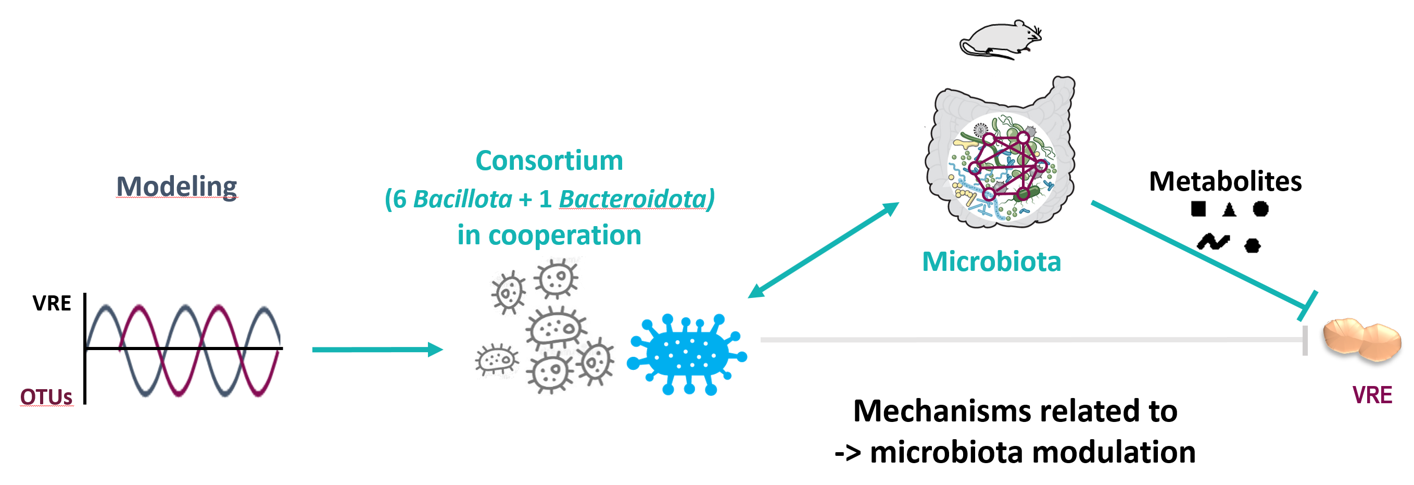
We aimed to identify commensal bacteria that play a key role in the ecological barrier effect to accelerate restoration of the gut microbiota after antibiotic dysbiosis and enhance resistance colonisation against VRE. We performed a longitudinal analysis of gut microbiota composition and VRE carriage levels during microbiota restoration in mice colonised with VRE after antibiotic-induced dysbiosis. By combining biological data and mathematical modeling, 15 molecular species (OTUs) negatively correlated with VRE carriage were identified. These species are potentially associated with a barrier effect against VRE. Seven representative strains of these OTUs were used in a mixture (Mix7) in two different mouse lines colonised with VRE. Mix7 reduces VRE carriage and promotes better recovery of the intestinal microbiota in so-called responder mice. These differences in the response of the mice were associated with variation in the initial microbiota, providing potential biomarkers for predicting response to Mix7. In addition, we demonstrated that the Bacteroidota strain is required for the Mix7 effect in vivo in the presence of at least one of the 6 strains. In a mouse model of persistent dysbiosis, the effect of Mix7 associated with higher concentrations of short-chain fatty acids (acetate, propionate, butyrate) and of 26 metabolites, including bile acids. None of the supernatants from the 7 strains, alone or in combination, inhibited VRE growth in vitro. Interestingly, 5 of the 7 strains are shared between humans and mice, and 2 have functional equivalents.
We have shown that supplementation with a mixture of strains identified by mathematical modeling improves the barrier effect against VRE through mechanisms dependent on the recovery and initial composition of the microbiota. Ultimately, this work will enable us to move towards personalised medicine by targeting at-risk patients likely to respond to supplementation with commensal anti-VRE strains, providing new live biotherapeutic products (LBPs) and biomarkers to predict response to treatment.
Contact:
Links:
Article: https://doi.org/10.1186/s40168-025-02127-5
Press: https://www.inrae.fr/en/news/using-bacteria-improve-microbiota-resistance-pathogens
Scoop.it Life Science Université Paris-Saclay: https://sco.lt/7ID9KC